最新進展
-
最新科研進展請參見實驗室新聞 http://old.chem.pku.edu.cn/xizf/revised/news.php?size=100&lang=
-
研究室工作進展 January 6, 2010Isolation and X-ray Structure of a Trimeric 1,4-Dilithio-1, 3-butadiene and a Dimeric Me3Si-substituted 1,4-dilithio-1,3-butadieneLantao Liu, Wen-Xiong Zhang,* Qian Luo, Heng Li, and Zhenfeng Xi*Organometallics 2010, 29, 278-281.
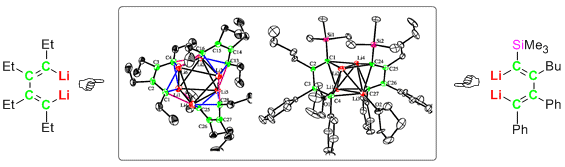 The X-ray crystallographic structures of a trimeric all-alkyl substituted 1,4-dilithio-1,3- butadiene with a Li6 pseudooctahedron as well as of the dimeric Me3Si-substituted 1,4-dilithio-1,3-butadiene with a Li4 tetrahedron are reported.
The X-ray crystallographic structures of a trimeric all-alkyl substituted 1,4-dilithio-1,3- butadiene with a Li6 pseudooctahedron as well as of the dimeric Me3Si-substituted 1,4-dilithio-1,3-butadiene with a Li4 tetrahedron are reported. 本工作背景介紹本研究小組自1999年發現1,4-雙锂-1,3-丁二烯衍生物具有不同與常見單锂金屬有機合成試劑的反應模式以來,一直努力建立合成該類衍生物純品的有效方法,以便最終将這類衍生物發展成方便使用的雙锂金屬有機合成試劑;同時,我們希望了解該類雙锂金屬有機化合物尤其是全烷基取代的衍生物的結構與成鍵模式。經過幾代研究生的努力,最終在2009年由劉瀾濤同學在張文雄老師的指導下完成該工作,可以方便地以80%以上的分離收率獲得克級以上多種衍生物的晶體(其中之一如圖),并作為試劑方便地用于進一步的反應。Background of this work1,4-Dilithio-1,3-butadienes found by our group in 1999 demonstrate different reaction patterns from commonly widely used mono-Li reagents. Since then, we have tried to establish an efficient synthetic method to get pure compounds, aiming at developing such compounds as useful organo-di-lithio reagents. After many trials by several graduate students, this goal was finally achieved in 2009 by Mr. Lantao Liu and Dr. Wen-Xiong Zhang. Most of such derivatives now can be obtained in this group as fine crystalline compounds in over 80% isolated yields with gram-scale. The picture shows one of such derivatives, which are kept in cooler and used when needed.
本工作背景介紹本研究小組自1999年發現1,4-雙锂-1,3-丁二烯衍生物具有不同與常見單锂金屬有機合成試劑的反應模式以來,一直努力建立合成該類衍生物純品的有效方法,以便最終将這類衍生物發展成方便使用的雙锂金屬有機合成試劑;同時,我們希望了解該類雙锂金屬有機化合物尤其是全烷基取代的衍生物的結構與成鍵模式。經過幾代研究生的努力,最終在2009年由劉瀾濤同學在張文雄老師的指導下完成該工作,可以方便地以80%以上的分離收率獲得克級以上多種衍生物的晶體(其中之一如圖),并作為試劑方便地用于進一步的反應。Background of this work1,4-Dilithio-1,3-butadienes found by our group in 1999 demonstrate different reaction patterns from commonly widely used mono-Li reagents. Since then, we have tried to establish an efficient synthetic method to get pure compounds, aiming at developing such compounds as useful organo-di-lithio reagents. After many trials by several graduate students, this goal was finally achieved in 2009 by Mr. Lantao Liu and Dr. Wen-Xiong Zhang. Most of such derivatives now can be obtained in this group as fine crystalline compounds in over 80% isolated yields with gram-scale. The picture shows one of such derivatives, which are kept in cooler and used when needed. -
研究室工作進展 Nov. 1, 2009One-pot Multicomponent Synthesis of Azaindoles and Pyrroles from OneMolecule of a Silicon-Tethered Diyne and Three or Two Molecules ofOrganonitriles Mediated by ZirconoceneShaoguang Zhang, Xiaohua Sun, Wen-Xiong Zhang, and Zhenfeng Xi*Chem. Eur. J. 2009, ASAP online October 31
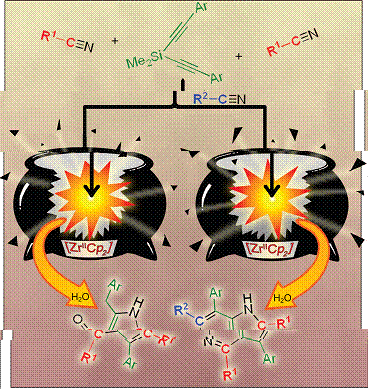 A zirconocene-mediated one-pot multi-component synthesis process has been developed, leading to the synthesis of 5-azaindoles and pyrrole derivatives, from a Si-tethered diyne and three or two different or identical organonitriles. 5-Azaindoles and pyrrole derivatives of diversified structures and substitution patterns could be highly selectively prepared via this protocol. A wide variety of organonitriles and Si-tethered diynes, either aliphatic or aromatic with both electron-withdrawing groups and electron-donating groups, could be used to afford 5-azaindoles and/or pyrroles in good-to-excellent isolated yields. A key intermediate formed in the Cp2Zr(II)-mediated reaction of one (PhC≡C)2SiMe2, two molecules of i-PrCN and one p-TolylCN was characterized by X-ray single-crystal structural analysis, which has allowed us to gain a good understanding of the reaction process.
A zirconocene-mediated one-pot multi-component synthesis process has been developed, leading to the synthesis of 5-azaindoles and pyrrole derivatives, from a Si-tethered diyne and three or two different or identical organonitriles. 5-Azaindoles and pyrrole derivatives of diversified structures and substitution patterns could be highly selectively prepared via this protocol. A wide variety of organonitriles and Si-tethered diynes, either aliphatic or aromatic with both electron-withdrawing groups and electron-donating groups, could be used to afford 5-azaindoles and/or pyrroles in good-to-excellent isolated yields. A key intermediate formed in the Cp2Zr(II)-mediated reaction of one (PhC≡C)2SiMe2, two molecules of i-PrCN and one p-TolylCN was characterized by X-ray single-crystal structural analysis, which has allowed us to gain a good understanding of the reaction process. -
Hydride-induced Novel Cyclization of Dienedinitriles Leading to Multi-functionalized CyclopentadienesHui-jun Zhang, Tianhao Meng, Bernard Demerseman, Christian Bruneau* and Zhenfeng Xi*Org. Lett. 2009, ASAP online.
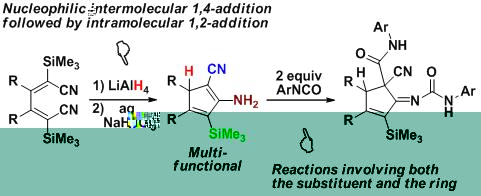
By treatment with LiAlH4, 1,4-dicyano-1,4-bis(trimethylsilyl)-1,3-dienes underwent a novel hydride-induced nucleophilic intermolecular 1,4-addition to the a, b-unsaturated nitrile moiety, followed by an immediate nucleophilic intramolecular 1,2-addition to the remaining CN group, to afford multiply functionalized cyclopentadienes in high isolated yields. These multi-functional (-CN, -NH2, -SiMe3, -H) cyclopentadienes are of a rich and interesting reaction chemistry as preliminarily demonstrated by their reaction with ArNCO involving both the substituent and the ring.
-
Isolation, Structural Characterization, and Synthetic Application of Oxy-cyclopentadienyl DianionsLantao Liu, Wen-Xiong Zhang, Chao Wang, Congyang Wang, and Zhenfeng Xi*Angew. Chem. Int. Ed. 2009, 48, in press.
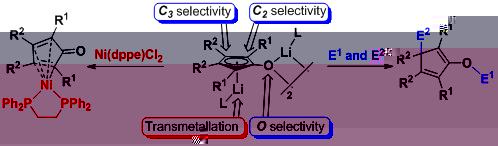 Isolation and applications of OCp: Novel cyclic dianions, lithio oxycyclopentadienyl dianions were isolated in high yields and structurally characterized by X-ray single crystal structural analysis. Interesting reaction chemistry and useful synthetic applications of these cyclic dianions were demonstrated to afford cyclopentadienes or transition-metal complexes.Cyclic dianions are very important synthetically useful intermediates for construction of organic compounds and organometallic complexes. However, synthetic methods and structural information for cyclic dianions are very much limited.This paper reports the first isolation and X-ray structural characterization of novel oxy-cyclopentadienyl (OCp for short) dianions generated via a concise reaction of 1,4-dilithio-1,3-butadienes with CO. The concomitance of the CpLi moiety, the exocyclic oxy anion, and those multi-reactive sites makes such dianion species structurally very unique and of novel reaction chemistry.
Isolation and applications of OCp: Novel cyclic dianions, lithio oxycyclopentadienyl dianions were isolated in high yields and structurally characterized by X-ray single crystal structural analysis. Interesting reaction chemistry and useful synthetic applications of these cyclic dianions were demonstrated to afford cyclopentadienes or transition-metal complexes.Cyclic dianions are very important synthetically useful intermediates for construction of organic compounds and organometallic complexes. However, synthetic methods and structural information for cyclic dianions are very much limited.This paper reports the first isolation and X-ray structural characterization of novel oxy-cyclopentadienyl (OCp for short) dianions generated via a concise reaction of 1,4-dilithio-1,3-butadienes with CO. The concomitance of the CpLi moiety, the exocyclic oxy anion, and those multi-reactive sites makes such dianion species structurally very unique and of novel reaction chemistry. -
Skeletal Rearrangement of All-carbon Spiro SkeletonsMediated by Lewis AcidFei Zhao, Chao Wang, Lantao Liu, Wen-Xiong Zhang, and Zhenfeng Xi*Chem. Commun. 2009, ASAP online.
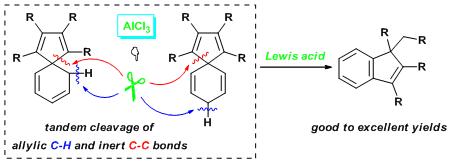 Mediated by AlCl3, substituted spiro[4.5]deca-tetraenesunderwent novel and selective skeletal rearrangement to generate indene derivatives in high to excellent yields.Selective cleavage of C-C and C-H bond is of great importance both fundamentally and practically. Sequential cleavage of both C–C and C–H bonds in all-carbon cyclic compounds spiro[4.5]deca-tetraenes was achieved to generate indene derivates via a novel AlCl3-mediated process.
Mediated by AlCl3, substituted spiro[4.5]deca-tetraenesunderwent novel and selective skeletal rearrangement to generate indene derivatives in high to excellent yields.Selective cleavage of C-C and C-H bond is of great importance both fundamentally and practically. Sequential cleavage of both C–C and C–H bonds in all-carbon cyclic compounds spiro[4.5]deca-tetraenes was achieved to generate indene derivates via a novel AlCl3-mediated process.This work has the following two features. (1) First example on the cleavage of C-C and C-H bond of all-carbon spiro compounds; (2) A new protocol on cleavage of C-C and C-H bond.
-
Zirconium- and Silicon-Containing Intermediates with Three Fused Rings in a Zirconocene-Mediated Intermolecular Coupling ReactionWen-Xiong Zhang, Shaoguang Zhang, Xiaohua Sun, Masayoshi Nishiura,Zhaomin Hou,* and Zhenfeng Xi*Angew. Chem. Int. Ed. 2009, 48, 7227-7231.
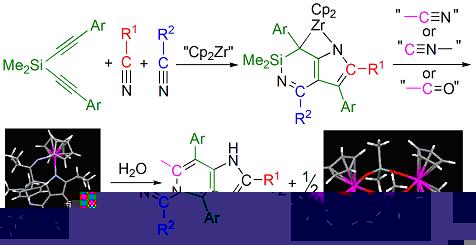 Veritable Jack-in-the-box: The isolation and characterization of key Zr/Si-containing intermediates was achieved for a zirconocene-mediated intermolecular coupling reaction (see scheme). The fate of the various functional groups was determined, and the reactivity of intermediate with a variety of unsaturated substrates was also investigated.This work demonstrates that the isolation and reactivity investigation ofreactive organometallic intermediates are not only of importance and interest for the in-depth understanding of seemingly complicated reaction mechanisms,but can alsolead to the discovery of new synthetic methods.
Veritable Jack-in-the-box: The isolation and characterization of key Zr/Si-containing intermediates was achieved for a zirconocene-mediated intermolecular coupling reaction (see scheme). The fate of the various functional groups was determined, and the reactivity of intermediate with a variety of unsaturated substrates was also investigated.This work demonstrates that the isolation and reactivity investigation ofreactive organometallic intermediates are not only of importance and interest for the in-depth understanding of seemingly complicated reaction mechanisms,but can alsolead to the discovery of new synthetic methods. -
Zirconocene-mediated ligand-switched selective cleavage of active and inert carbon-carbon bonds in allylcyclopropanes
Chao Wang, Liang Deng, Jun Yan, Hailin Wang, Qian Luo, Wen-Xiong Zhang and Zhenfeng Xi*Chem. Commun., 2009, 4414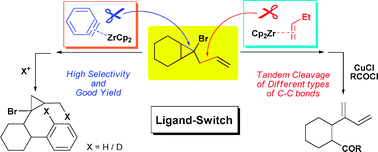 In this paper, we have developed a novel zirconium (II)-mediated ligand-controlled selective cleavage of different C–C bonds in bromoallylcyclopropane, which contains multiply reactive sites. This reaction represents a new way leading to selective cleavage of both reactive and inert C–C bonds.本論文主要研究了低價有機锆試劑促進的多取代環丙烷的開環反應。在反應中對锆的配體進行調控後,惰性的環丙烷側鍊碳碳鍵可以被切斷,而活潑的三元母環卻可以保持。碳碳鍵活化以其重要性和高難度被稱為化學的聖杯。本部分的工作初步體現了一類新型的碳碳鍵活化過程。
In this paper, we have developed a novel zirconium (II)-mediated ligand-controlled selective cleavage of different C–C bonds in bromoallylcyclopropane, which contains multiply reactive sites. This reaction represents a new way leading to selective cleavage of both reactive and inert C–C bonds.本論文主要研究了低價有機锆試劑促進的多取代環丙烷的開環反應。在反應中對锆的配體進行調控後,惰性的環丙烷側鍊碳碳鍵可以被切斷,而活潑的三元母環卻可以保持。碳碳鍵活化以其重要性和高難度被稱為化學的聖杯。本部分的工作初步體現了一類新型的碳碳鍵活化過程。 -
Synthesis of functionalized siloles from Si-tethered diynes
Qian Luo, Li Gu, Chao Wang, Junhui Liu, Wen-Xiong Zhang and Zhenfeng Xi*Tetrahedron Lett. 2009, 32, 3213-3215(Tetrahedron Letters 創刊五十周年紀念專集邀請論文) A new synthetic itinerary to silole from Si-tethered diynes is reported. In this protocol, the Si-tethered diyne manifests definitely the reactivity of monoyne to form the lithio silole via zirconacyclopetadiene, 1,4-diiodo-1,3-butadiene and the corresponding dilithiodiene successively. Lithio siloles thus obtained above could be easily functionalized to give various types of silole derivatives. Complex structure like bridged bis-silole compounds could also be constructed by this process.噻咯 (Silole)在材料化學研究領域非常重要。本文以矽橋連二炔為起始物發展了合成噻咯的新方法。矽橋連二炔以單炔的反應性通過锆雜環戊二烯,相應的1,4-二碘-1,3-丁二烯以及1,4-二锂-1,3-丁二烯這樣的反應過程最終形成了锂代噻咯。锂代噻咯可以被很容易地進一步官能團化,從而形成不同的噻咯衍生物。通過此方法,可以得到用其它方法很難得到的結構複雜的橋連雙噻咯化合物。
A new synthetic itinerary to silole from Si-tethered diynes is reported. In this protocol, the Si-tethered diyne manifests definitely the reactivity of monoyne to form the lithio silole via zirconacyclopetadiene, 1,4-diiodo-1,3-butadiene and the corresponding dilithiodiene successively. Lithio siloles thus obtained above could be easily functionalized to give various types of silole derivatives. Complex structure like bridged bis-silole compounds could also be constructed by this process.噻咯 (Silole)在材料化學研究領域非常重要。本文以矽橋連二炔為起始物發展了合成噻咯的新方法。矽橋連二炔以單炔的反應性通過锆雜環戊二烯,相應的1,4-二碘-1,3-丁二烯以及1,4-二锂-1,3-丁二烯這樣的反應過程最終形成了锂代噻咯。锂代噻咯可以被很容易地進一步官能團化,從而形成不同的噻咯衍生物。通過此方法,可以得到用其它方法很難得到的結構複雜的橋連雙噻咯化合物。
-
Star-shaped Silacyclobutene-Containing π-Systems:Synthesis and Optical PropertiesOrganometallics 2009, 28, 413-417
 Well-defined π-conjugated systems containing two or three silacyclobutene units have been synthesized in high yields via regioselective coupling of bis(phenylethynyl)silanes with dialkynylbenzene or trialkynylbenzene in the presence of Cp2ZrBu2. The UV-vis absorption and fluorescence spectra of these new π-conjugated systems have demonstrated that the increase in silacyclobutene units per molecule from two or three brings about an increase of the extinction coefficient.從單炔基苯、二炔基苯或三炔基苯以及二炔基矽烷出發,以較高産率合成了以苯為核、結構中含有一個、兩個和三個矽雜環丁烯骨架的共轭有機矽分子。随着分子中矽雜環丁烯骨架的增加,這類化合物的紫外吸收與熒光效率均有顯著的提高。
Well-defined π-conjugated systems containing two or three silacyclobutene units have been synthesized in high yields via regioselective coupling of bis(phenylethynyl)silanes with dialkynylbenzene or trialkynylbenzene in the presence of Cp2ZrBu2. The UV-vis absorption and fluorescence spectra of these new π-conjugated systems have demonstrated that the increase in silacyclobutene units per molecule from two or three brings about an increase of the extinction coefficient.從單炔基苯、二炔基苯或三炔基苯以及二炔基矽烷出發,以較高産率合成了以苯為核、結構中含有一個、兩個和三個矽雜環丁烯骨架的共轭有機矽分子。随着分子中矽雜環丁烯骨架的增加,這類化合物的紫外吸收與熒光效率均有顯著的提高。
