研究室工作進展 Aug. 15th, 2015
1,3-Butadienyl Dianions as Non-innocent Ligands: Synthesis and Characterization of Aromatic Dilithio Rhodacycles
Junnian Wei, Yongliang Zhang, Wen-Xiong Zhang, and Zhenfeng Xi*
Angew. Chem. Int. Ed. 2015, 54, 9986-9990.(Very Important Paper)
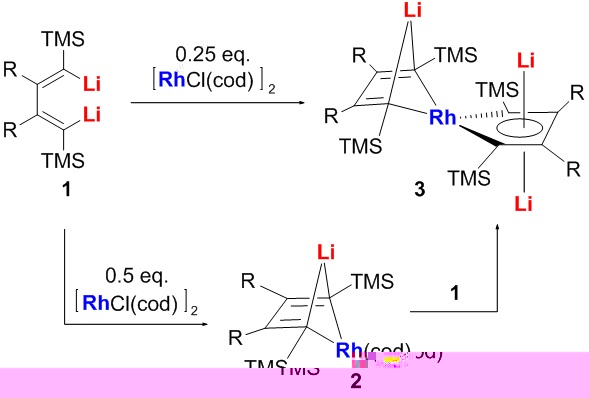
Recently we reported the first example that organolithium reagents might behaved as formal oxidants (Angew. Chem. Int. Ed. 2015, 54, 5999-6002). In that reaction, 1,4-dilithio-1,3-butadienes (dilithio reagents for short) reacted with Ni(cod)2, offering aromatic dilithionickeloles as final products. Ni(0) was assumably oxidized to Ni(II) by the dilithio reagents based on XPS data. Excited by this fascinating discovery and at the meantime as our conclusion seems to have conflicts with classic theory of formal oxidation state formalism, we tried to have more examples and evidences to discuss this topic more deeply and systematically.
Here we report that 1,4-dilithio-1,3-butadienes, a type of 1,3-butadienyl dianions, can act as non-innocent ligands, taking electrons from low-valent transition metals. Dilithio reagents reacted with [RhCl(cod)]2 to offer dilithio rhodacycle. Single-crystal X-ray structural analysis revealed the structure with averaged bond lengths. XPS data suggested that the oxidation state of Rh was more likely to be Rh3+. CDA/ECDA confirmed the electron transfer process. 7Li NMR spectra and theoretical calculations revealed a considerable aromatic character. In this process, the dilithio compounds behaved as non-innocent ligands and formal oxidants. These results further demonstrated that organolithium compounds with suitable π-conjugation could be used as electron acceptor.
亮點介紹
碳負離子可以作為氧化劑繼續接受電子
在已知的氧化還原反應中,碳負離子如常見的有機锂試劑可能作為還原劑而失去電子,但是不作為氧化劑進一步得到電子。本課題組魏俊年同學利用我們自己發展的雙锂試劑及其協同效應,與低價過渡金屬配合物如Ni(cod)2和[RhCl(cod)]2反應,合成了相應的芳香性金屬雜環戊二烯衍生物(For Ni,see: Angew. Chem. Int. Ed. 2015, 54, 5999-6002)。在該反應中,雙锂試劑的丁二烯基雙碳負離子共轭體系的反鍵軌道與低價過渡金屬的d軌道中的一對電子産生“協同效應”,使富電荷的丁二烯骨架繼續獲得電子,成功構建了具有芳香性的金屬雜環戊二烯衍生物。該研究打破了傳統所認為的碳負離子不能夠繼續獲得電子的認知,為共轭體系碳負離子化學的進一步發展和應用提供了一個全新的思路。
單晶結構和理論計算證實了所生成的金屬雜環戊二烯衍生物具有顯著的芳香性。X-射線光電子能譜顯示過渡金屬的氧化态顯著上升(Ni近似+2價,Rh近似+3價)。由于雙锂試劑中碳負離子與丁二烯骨架的協同效應,其LUMO軌道能級相對于1,3-丁二烯顯著降低,故一些過渡金屬的d軌道電子可離域至雙锂試劑的π*軌道,完成芳香性金屬雜環的構建。雙锂試劑在該反應中可視為一類特殊的non-innocent ligand或者redox-active ligand,發生了氧化還原反應。
該系列工作也是本課題組雙金屬有機合成試劑化學的新展開。
